A research team led by Prof. SHEN Wenjie, in collaboration with Prof. LIU Jingyue from Arizona State University, identified the key role of the geometrical structure of the Au-Fe2O3 interfaces in CO oxidation. Their findings were published in Angew. Chem. Int. Ed..
Geometrical interfacial structure of Au-Fe2O3 catalyst. (Image by WANG Ning)
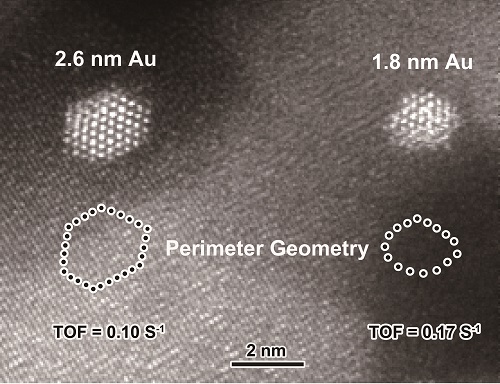
Gold nanoparticles (NPs), dispersed on reducible metal oxides, are frequently reported to be highly active for low-temperature oxidation of CO, showing a prominent size effect. Au NPs smaller than 3 nm are highly active while Au NPs larger than 5 nm are nearly inert. However, the chemical nature of the size-dependence remains controversial.
The researchers investigated the atomic arrangement of the perimeter gold atoms at the Au-Fe2O3 interfaces by tuning the size of Au NPs in the range of 2-5 nm, using aberration corrected scanning transmission electron microscopy.
They found that the activity for CO oxidation on Au/Fe2O3 catalysts was intimately associated with the geometrical structure of the interfacial perimeter Au atoms. For smaller gold particles, the perimeter gold atoms possessed a low-coordinated environment and thus showed a much higher intrinsic activity.
This result extends the fundamental understanding of the size effect in nanocatalysis and provides a promising strategy for tuning the metal-oxide interactions.
The research was supported by National Natural Science Foundation of China. (Text by WANG Ning)