A research team led by Prof. BAO Xinhe and Prof. WANG Guoxiong enhanced CO2 electrolysis performance with Vanadium-doped perovskite cathode in solid oxide electrolysis cell (SOEC). Their findings were published in Nano Energy.
High-temperature CO2 electrolysis using SOECs is capable of reducing CO2 and water to syngas and hydrocarbon fuel at the cathode, while producing pure oxygen at the anode. Due to the advantages of possessing solid and modular structure, high energy efficiency and low cost, SOECs have potential applications in CO2 conversion and surplus sustainable electricity storage.
The development of perovskite oxides as cathode materials in SOEC is a hot research area in recent years due to its stable structure and effective inhibition of carbon deposition in a redox atmosphere. However, perovskite oxides electrodes still have great challenges in CO2 adsorption, activation and conversion, resulting in a low performance of electrocatalytic reduction of CO2.
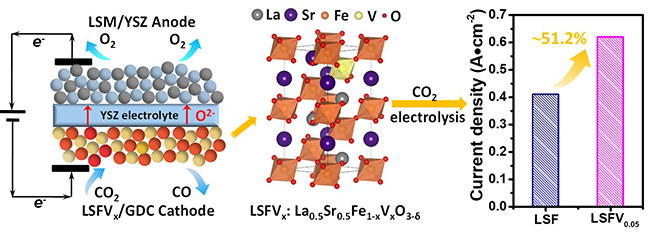
Enhanced performance of high-temperature CO2 electrolysis over vanadium-doped perovskite cathode in SOEC. (Image by ZHOU Yingjie and ZHOU Zhiwen)
In this work, researchers investigated the nanocomposites of La0.5Sr0.5Fe1-xVxO3-δ with Ce0.8Gd0.2O1.9 (LSFVx/GDC) as cathode materials for pure CO2 electrolysis in SOEC.
The introduction of vanadium promotes the formation of oxygen deficiencies in LSFVx/GDC, thus greatly enhanced CO2 adsorption and dissociation, as well as the electrocatalytic performance. The highest current density of 0.62 A cm-2 could be achieved over the LSFV0.05/GDC cathode at 1.6 V, which was about 51.2% increase compared with that over the LSF/GDC cathode.
This study regulated oxygen vacancy concentration and CO2 activation/adsorption ability of SOEC cathode materials via doping metal element, which provided a new strategy to improve the performance of electrocatalytic reduction of CO2 at the SOEC cathode.
This work was financially supported by Natural Science Foundation of China, National Key R&D Program of China, Dalian Institute of Chemical Physics and Strategic Priority Research Program of the Chinese Academy of Sciences. (Text by ZHOU Yingjie and ZHOU Zhiwen)