Research groups led by Prof. YANG Qihua and Prof. LIU Jian promoted confined catalysis in nanoreactors. Their results were published in Angewandte Chemie International Edition as inside cover and selected as VIP paper.
The catalytic performance of enzyme is directly related with the environment around the active sites. It is a challenging task to promote the activity and selectivity of a catalyst via precisely engineering the microenvironment.
The catalytic hydrogenation of benzoic acid (BA) to cyclohexanecarboxylic acid is of pivotal importance in industry. However, metal nanoparticles (NPs) showed low activity for this reaction, especially in aprotic solvent. In order to promote the catalytic activity of metal NPs, scientists introduced ruthenium NPs into nanoreactors modified with organic ligands (eg. amino, triphenylphosphine, diphenylphosphine, phenyl).
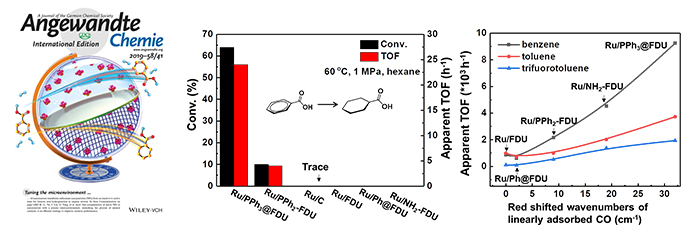
Left: The catalytic performance of Ru NPs in hydrogenation of BA using hexane as solvent. Right: Relation of apparent TOF with the red shifted wavenumbers of linearly adsorbed CO of Ru NPs in hydrogenation of benzene, toluene, and trifluorotoluene under neat conditions. (Image by REN Xiaomin and GUO Miao)
The catalytic results demonstrated that the Ru NPs in phosphine ligand modified nanoreactors could effectively catalyze the hydrogenation of benzoic acid in hexane, no conversion of BA could be found over Ru/NH2-FDU, Ru/Ph@FDU, Ru/FDU and commercial Ru/C.
Both density functional theory (DFT) calculations and catalytic performance tests showcased that the phosphine ligands could manipulate the adsorption strength of BA on Ru NPs by tuning the surface properties as well as preferentially interacting with the carboxyl of BA. At the same time, the scientists demonstrated the nanoreactors modified with phosphine ligand could promote the activity in the hydrogenation of benzene, toluene and trifluorotoluene owing to the negatively charged surface of metal.
The insights obtained in this study provides a novel concept of nanoreactor design via anchoring ligands near catalytic active centers.
The work was supported by the National Natural Science Foundation of China and the Strategic Pilot Project of the Chinese Academy of Sciences. It also dedicated to the 70th anniversary of DICP. (Text by REN Xiaomin and GUO Miao)