A joint research team led by Prof. XIAO Jianping from SKLC, as well as Prof. XIAO Fengshou and Prof. WANG Liang from Zhejiang University has reported a study for selective ethanol production, of which the conversion and ethanol selectivity reach up to 42.4% and 35.5%, respectively. This work was published in Chem on Jan. 16.
Ethanol is an important chemical and fuel. Direct and efficient conversion from syngas (CO and H2) to ethanol is in great demand, but faces challenges due to low selectivity.
The new method was realized through a novel RhMn@S-1 catalyst (the RhMn catalysts confined in an S-1 zeolite). The experiments showed that S-1 zeolite could stabilize the Mn-O-Rhδ+ structure, which is vital for ethanol production.
However, the mechanism of how Mn-O-Rhδ+ structure selectively produce ethanol is still obscure. Therefore, a deep understanding of the nature of catalytic selectivity and activity is also significant for improving the design of catalysts.
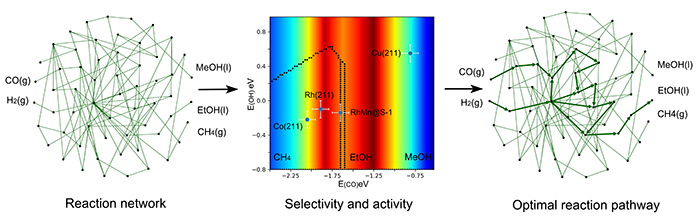
Reaction network and phase diagram. (Image by QIN Gangqiang)
“There are dozens of intermediates and transition states along the process of syngas conversion to methane, methanol, and ethanol, resulting in thousands of possible reaction pathways. We need a general physical picture to guide our thinking and design,” said Prof. XIAO.
Traditional methods cannot provide an intuitive view to understand the selectivity and activity of various products. The reaction network and potential surfaces can be simplified by the method developed by Prof. XIAO’s group, which is the so-called 2D reaction phase diagram based on two descriptors.
It is wildly accepted that methane is the main products on pure Rh and Co metal because of full breaking of C-O bond. However, the metals with weak CO reactivity exhibit CH3OH selectivity, such as Cu.
“Our method has found the most feasible pathway, where the C-C coupling between CO/CHO* and CH2* species is determining for ethanol production. That means the ethanol production rate reaches to maximum as a half of the CO dissociates. In other words, we need a less reactive catalyst compared to conventional Rh particles,” said Prof. XIAO.
The theoretical study rationalizes well the experimental observation for syngas conversion to ethanol. The concept and scenario in this study can accelerate rational design and computational screening of new catalysts in the future. (Text by QIN Gangqiang)