A research team led by Prof. PAN Xiulian and Prof. BAO Xinhe from the State Key Laboratory of Catalysis(SKLC) discovered dynamic confinement of SAPO-17 cages on the selectivity control of syngas conversion.
This study was published in National Science Review on July 26.
In 2016, the team proposed a new catalyst concept based on metal oxide-zeolite bifunctional catalysts (OXZEO), which enabled the direct conversion of syngas to light olefins with high selectivity.
In this study, the researchers reported the dynamic confinement effect of zeotype cages, which controlled the product selectivity during the induction period of syngas conversion. They increased the ethylene selectivity from 19% gradually to 44% whereas decreased C4+ hydrocarbon selectivity from 39% to 9% within the first 22 hours on stream. After the induction period, the catalytic performance leveled off.
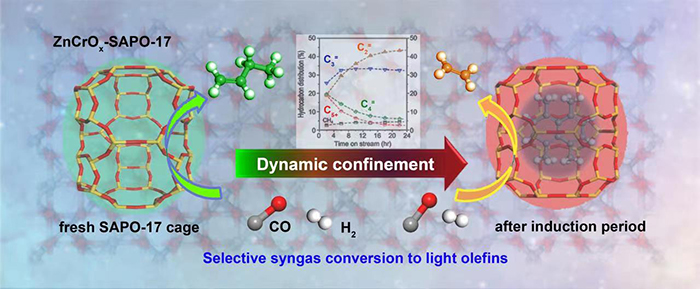
Dynamic confinement of SAPO-17 cages on the selectivity control of syngas conversion (Image by WANG Haodi and JIAO Feng)
By the characterization with structured illumination microscopy, intelligent gravimetric analysis, UV-Raman, X-ray diffraction, thermogravimetry, and gas chromatography-mass spectrometer analysis, they indicated that this was induced by the gradual accumulation of carbonaceous species inside the SAPO-17 cages as the reaction proceeds. It led to a gradually decreased free space inside the cage.
They found that the diffusion coefficient ratio of C2 to C4 was correlated negatively with an Effective Space Coefficient (ESC), a descriptor that was defined to describe the effective space inside the SAPO-17 cage. It indicated more hindered diffusion for C4 than for C2 with the reduced free space of the cage. Furthermore, a restricted free space would also hinder the secondary reaction of ethylene and therefore benefited C2 selectivity.
"This study reveals a significant effect of the dynamic confinement of SAPO-17 cage on the product selectivity. Although the most of micropores are occupied (93%) when the induction period is completed, the catalyst is not deactivated and it is running rather stably in syngas conversion," said Prof. PAN.
This dynamic confinement is expected to be general for a number of reactions involving hydrocarbons over zeolites. The understanding is essential for further design of high-performance zeolites-based catalysts for C1 chemistry as well as other reactions involving hydrocarbons.
This work was supported by the Ministry of Science and Technology of China, the National Natural Science Foundation of China, the Youth Innovation Promotion Association of CAS, Dalian Science and Technology Innovation Fund, the Natural Science Foundation of Liaoning. (Text by WANG Haodi and JIAO Feng)