Formic acid and acetic acid are pivotal platform chemicals. Distillation-based water/acid separation requires large energy expenditure.
Separation using membranes is identified to be a low-carbon technology. Metal-organic frameworks (MOFs) bring tremendous opportunities for membrane separation. Highly compact membranes benefit both separation accuracy and long-term stability. However, the preparation of such membranes for upgrading of corrosive acids is still challenging.
Recently, a research group led by Prof. YANG Weishen and Dr. BAN Yujie from the State Key Laboratory of Catalysis(SKLC) proposed a "template-guiding growth" method to prepare a highly compact MIL-53 (a class of MOF) membrane.
"This membrane can achieve complete dewatering from formic acid and acetic acid solutions, and save up to 77% of the energy consumption compared with the traditional distillation," said Dr. BAN
This study was published in Angewandte Chemie International Edition on Feb. 22.
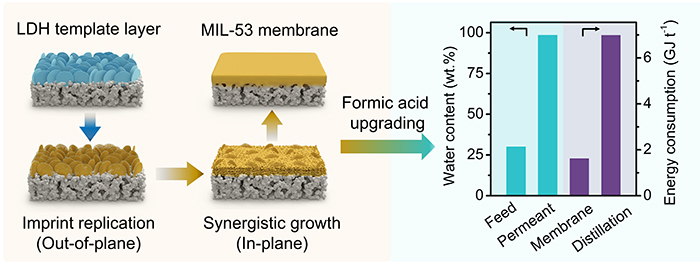
A layered double hydroxide nanoflake template layer triggers conversion into a highly compact MIL-53 membrane (Image by BAN Yujie and WANG Yuechen)
The researchers reported that a continuous layer of ZnAl-CO3 layered double hydroxide nanoflakes on an alumina supported as a template triggered chemical self-conversion to MIL-53 membrane.
In this process, the template guided MIL-53 crystals to replicate its imprint out-of-plane (stage 1) and modulated the availability of al from the alumina support for in-plane growth of MIL-53 (stage 2). The membrane could realize nearly complete dewatering from formic acid and acetic acid solutions, respectively, and maintain stability in a continuous pervaporation.
This work was supported by the National Natural Science Foundation of China.