Proton exchange membrane (PEM) water electrolysis is a significant technology for producing large-scale green hydrogen. While Platinum on Carbon (Pt/C) is a state-of-the-art cathode catalyst due to its moderate hydrogen binding energy and high resistance to acid corrosion, its high Pt loadings significantly increase costs.
Schematic illustration for confinement of single-site Pt by the enriched asymmetric electrons with boosted activity and stability for acidic HER (Image by XU Mingxia)
In a study published in Joule, a research team led by Prof. DENG Dehui and Prof. YU Liang from the State Key Laboratory of Catalysis (SKLC) , in collaboration with Prof. LU Junling from the University of Science and Technology of China (USTC) and Prof. YU Hongmei from the DICP, developed a highly efficient and stable catalyst for acidic hydrogen evolution. This catalyst employs enriched asymmetric π electrons on the surface of a chainmail structure to create a unique confinement effect, enhancing both the activity and stability of surface-confined platinum (Pt) atoms.
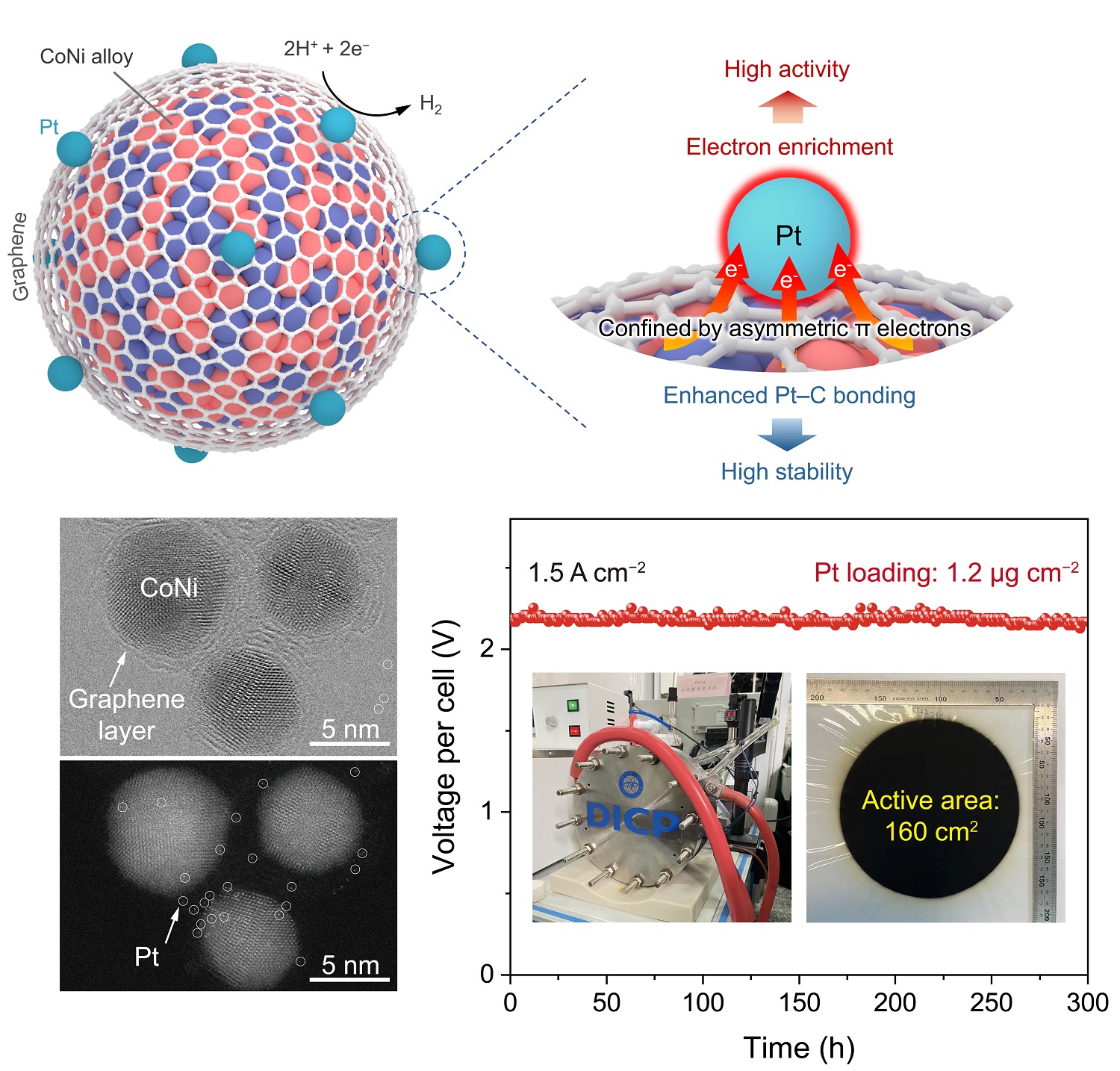
The researchers designed a chainmail catalyst consisting of a cobalt-nickel (CoNi) nano-alloy encapsulated in a monolayer of graphene. They discovered that electron transfer from CoNi to the carbon layer, along with 3d-2p electronic interaction, led to an enrichment of asymmetric π electronic states on the graphene surface. After depositing Pt single atoms using atomic layer deposition, these enriched asymmetric π electrons exhibited a unique confinement effect on the Pt atoms.
This confinement operates through two synergistic mechanisms: First, electron transfer from CoNi to Pt via the graphene layer results in an electron-rich Pt site, optimizing hydrogen adsorption energy and promoting hydrogen desorption, thereby improving catalytic activity. Second, strong interactions between the asymmetric π electrons and the Pt 5d orbital enhance the structural stability of Pt sites, boosting the durability of the catalyst.
Furthermore, the researchers assembled a proton exchange membrane (PEM) water electrolyzer with this catalyst. It achieved an ultra-high current density of 4.0 A cm−2 at 2.02 V and maintained excellent durability over 1,000 hours at 2 A cm−2, using only 1.2 μgPt cm−2 Pt loading. Moreover, they assembled a 2.85 kW PEM water electrolyzer using this catalyst operated stably for over 300 hours at an industrial current density of 1.5 A cm−2, highlighting its outstanding industrial application potential.
"This work provides a new idea for developing high-performance, long-life and low-cost catalysts for hydrogen production via acid water electrolysis," said Prof. DENG.